Ca1−xSrxRuO3 perovskite at the metal–insulator boundary as a highly active oxygen evolution catalyst†
Abstract
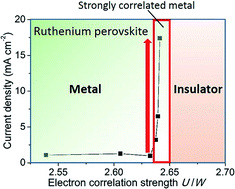
The oxygen evolution reaction (OER) plays a key role in the electrode reactions of emerging energy conversion technologies such as water splitting and rechargeable metal–air batteries. Herein, we report that stronger electron–electron correlation leads to higher OER activity by a systematic electrochemical study of Ca1−xSrxRuO3. Specifically, we find that when the conducting material is sufficiently near the metal–insulator boundary (Ca0.9Sr0.1RuO3 in this study), it leads to a remarkable activity enhancement by a factor of 17. Our finding suggests that electron correlation not only enhances the initial OER activity but also stabilizes the structure of the catalyst. Therefore, the coexistence of metallic conductivity and strong electron correlation is beneficial for developing highly active OER catalysts. Furthermore, the excellent OER activity at x ∼ 0.1 is accompanied by superior bifunctionality toward the OER and the oxygen reduction reaction (ORR). Our study also revealed that conducting materials close to the metal–insulator boundary are promising oxygen reaction catalysts for energy conversion technologies.


 Please wait while we load your content...
Please wait while we load your content...