Discovery of a new intercalation-type anode for high-performance sodium ion batteries†
Abstract
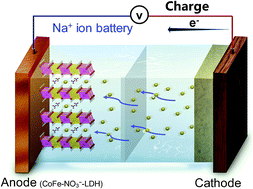
A CoFe layered double hydroxide (LDH) with nitrates in its interlayer has been developed for the first time as an intercalation-type anode material for sodium ion batteries (SIBs). Unlike the well-recognized conversion reaction for metal hydroxides in lithium ion batteries, an exceptional intercalation/de-intercalation mechanism for Na+-storage was revealed. The CoFe–NO3−-LDH anode delivers an initial discharge capacity of 498 mA h g−1 and a reversible capacity of 209 mA h g−1 after 200 cycles at 1 A g−1. The superb Na+-storage performance is attributed to the expanded interlayer distance of the CoFe-LDH pillared by the interlayer nitrates, benefiting the intercalation/de-intercalation of Na+ ions. Besides, the topochemical transformation property of LDH materials guarantees high structural stability during the valence state change of Co2+/Co2−x and Fe2+/Fe3+ in their host layers. In addition, theoretical calculation reveals a low Na+ diffusion barrier of 0.147 eV in the interlayer space of CoFe–NO3−-LDH, which manifests LDH as a good Na+ ion conductor. Therefore, this work not only develops a novel intercalation-type anode for SIBs, but also provides new insights into the energy-storage mechanism of layered metal hydroxides.


 Please wait while we load your content...
Please wait while we load your content...