Metallic NiSe2 nanoarrays towards ultralong life and fast Li2S oxidation kinetics of Li–S batteries†
Abstract
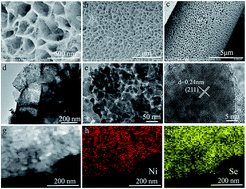
The complex solid–liquid–solid phase transition in Li–S batteries, the serious shuttle effect of soluble polysulfides, sluggish polysulfide conversion kinetics and the low conductive nature of Li2S cause a high decomposition barrier, inevitably limiting the development of advanced Li–S batteries. In addition, the severe sedimentation problem of nonconductive Li2S during the charging process would inevitably hamper the subsequent reaction kinetics of Li–S batteries, especially at high sulfur loading. Herein, this paper, for the first time, reports the construction of a highly conductive sulfur host material (C@NiSe2) by simulating the decomposition process of Li2S on the surface of anchor materials, which confirmed that NiSe2 is more suitable for the Li2S oxidation process. In addition, the strong binding strength of NiSe2 with Li2S is better for the decomposition of Li2S during the charge process. Therefore, when C@NiSe2 is used as the electrode material, the batteries exhibited an initial capacity of 1250 mA h g−1 with a quite low cycle decay of 0.089% per cycle with a sulfur loading of 5 mg cm−2 at 0.2C. At a high current density of 5C, the battery showed an ultralow cycle decay of 0.019% during 2000 cycles. In addition, with an ultrahigh sulfur loading of 12.0 mg cm−2, the C@NiSe2/S electrode could still deliver a significant areal capacity of 9.96 mA h cm−2, which is good for realizing practical applications of Li–S batteries.


 Please wait while we load your content...
Please wait while we load your content...