Directional oxygen activation by oxygen-vacancy-rich WO2 nanorods for superb hydrogen evolution via formaldehyde reforming†
Abstract
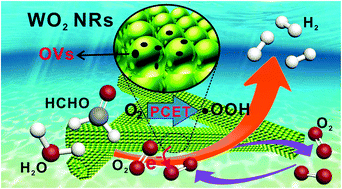
Molecular oxygen activation (MOA) into reactive oxygen species (ROS) is extremely crucial in numerous catalytic processes, while precise control of ROS products remains difficult. Herein, we find that oxygen substoichiometric tungsten dioxide nanorods (WO2 NRs) are an appropriate alternative to realize highly efficient O2 promoted HCHO reforming to produce H2. Rich oxygen vacancies (OVs) in WO2 NRs play a fundamental role in the MOA induced catalytic H2 evolution reaction because they guarantee the favorable chemisorption of molecular O2 on the catalyst surface as well as build up a channel for the delivery of electrons to O2 species. Importantly, MOA initiated by the WO2 NR catalyst can be achieved by systematically modulating the proton-coupled electron transfer (PCET) pathway to controllably obtain the desired ROS and thus to directionally produce H2, in which molecular O2 receives the electrons to form superoxide radicals in a chemisorbed state (O2˙−) and couple with protons provided by HCHO to convert into ·OOH radicals, rather than ·OH or other ROS. The surface stabilized HOO·–WO2 NR complex acts as the catalytically active center in the dehydrogenation reaction. As O2 continuously takes up and releases protons by formation and consumption of ·OOH radicals, O2 persistently circulates on the WO2 NR surface, making WO2 NRs an unconventional catalyst. This study develops a new technique for clean energy supply and deepens our understanding of the MOA process during heterogeneous catalysis.


 Please wait while we load your content...
Please wait while we load your content...