Monolayer GaS with high ion mobility and capacity as a promising anode battery material†
Abstract
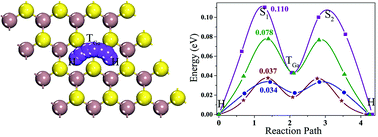
Layered gallium sulphide (GaS) with hexagonal symmetry is a typical metal mono-chalcogenide and is mechanically stable under tension. In this work, we used first-principles density functional theory to study the performance of monolayer (ML) GaS as an anode material for Li, Na, K, Mg, and Al-ion batteries. The most stable binding sites for the metal atoms on ML GaS are all in the hollow sites, and the Fermi level of ML GaS moves upward into the conduction band after adsorption of Li, Na, K, and Al atoms, showing a semiconductor-to-metal transition of ML GaS, with the exception of Mg atoms. The binding energies of the metal atoms with ML GaS are all negative at all the studied densities, indicating a steady adsorbing process. The Li, Na, K, and Al ions prefer to move along the zigzag direction on ML GaS, with relatively low diffusion barrier heights of 0.110, 0.078, 0.037, and 0.034 eV, respectively. Such low diffusion barriers endow ML GaS with high rate capacity. The maximum theoretical specific capacities for Li, Na, K, and Al-ion batteries are 526.74, 32.92, 32.92, and 98.76 mA h g−1, respectively. Noticeably, the theoretical specific capacity of ML GaS for Li-ion batteries (LIBs) is even higher than the theoretical one (372 mA h g−1) of commercially used graphite. Besides, an appropriate average open circuit voltage of 0.53 V is generated for ML GaS based LIBs. Our study suggests that ML GaS is a suitable anode material for LIBs.


 Please wait while we load your content...
Please wait while we load your content...