Nickel based oxide film formed in molten salts for efficient electrocatalytic oxygen evolution†
Abstract
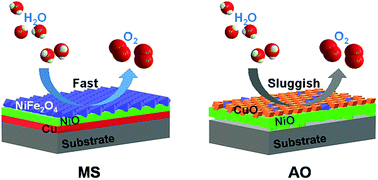
Implementation of non-precious, active and durable catalysts towards the oxygen evolution reaction (OER) is a promising protocol addressing challenges in energy and environmental sustainability. Herein, a binder-free film composed of nickel ferrite is prepared by the anodic oxidation of a nickel alloy in molten salts. Merits of the electrochemical oxidation in molten salts are highlighted by the generation of the spinel NiFe2O4 film over the Ni–Fe–Cu substrate with full exposure of OER-active Fe species, absence of OER-inert CuO species and good adhesion with the substrate. The prepared film electrode therefore exhibits higher electrocatalytic activity than the commercial IrO2/Ta2O5–Ti electrode towards the OER in an alkaline electrolyte. The overpotential to drive a current density of 10 mA cm−2 for the as-prepared electrode is only 326 mV, 27 mV less than that for the IrO2/Ta2O5–Ti electrode. Besides, the prepared nickel ferrite film also exhibits a low Tafel slope of 35.9 mV dec−1, an excellent turnover frequency (TOF) value of 0.85 s−1 at an overpotential of 300 mV and high stability after electrolysis at 50 mA cm−2 for 60 h in 1.0 M KOH. The present study highlights the potential for utilization of electrochemical oxidation of metallic substrates in molten salts for the fast preparation of high-quality oxide films with promising functionalities.
- This article is part of the themed collection: 2019 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...