Ammonium chloride–metal hydride based reaction cycle for vehicular applications†
Abstract
Hydrogen and ammonia have attracted attention as potential energy vectors due to their abundance and minimal environmental impact when used as a fuel source. To be a commercially viable alternative to fossil fuels, gaseous fuel sources must adhere to a wide range of standards specifying hydrogen delivery temperature, gravimetric capacity and cost. In this article, an ammonium chloride–metal hydride reaction cycle that enables the solid thermal decomposition products to be recycled using industrial processes is proposed. A range of metal hydrides and metal amides were reacted with ammonium chloride to determine the reaction pathways, products and overall feasibility of the cycle. The NH4Cl–MH (MH = metal hydride) and NH4Cl–MNH2 (MNH2 = metal amide) mixtures were heated to temperatures of up to 500 °C. The resulting products were experimentally characterised using temperature program desorption residual gas analysis, simultaneous differential scanning calorimetry and thermogravimetric analysis and in situ powder X-ray diffraction. Similar analysis was undertaken to determine the effect of catalyst addition to the starting materials. A maximum yield of 41 wt% of hydrogen and ammonia gas mixtures were released from the NH4Cl–MH materials at a maximum yield of 41 wt%. This exceptional gravimetric capacity allows for volumetric gas densities (363–657 kg m−3) that are much higher than pure NH3, H2 or metal hydride materials. Overall, this reaction cycle allows carbon-neutral regeneration of the starting materials, making it a potential sustainable energy option.
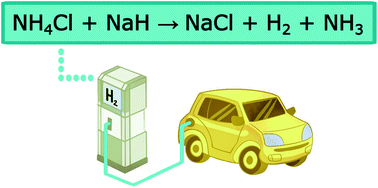


 Please wait while we load your content...
Please wait while we load your content...