Polydopamine-induced surface functionalization of carbon nanofibers for Pd deposition enabling enhanced catalytic activity for the oxygen reduction and evolution reactions†
Abstract
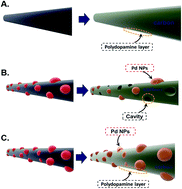
A detailed understanding of the surface modification or coating of materials is becoming more important for the design and development of hybrid materials for their advanced applications. The characteristics of polydopamine-coated surfaces were explored by varying dopamine concentration and polymerization time for noble metal deposition, which is a key for the development of advanced catalysts. The variation of these parameters for dopamine coating on carbon nanofibers (CNFs) finally modulated the amount of palladium (Pd) nanoparticles deposited on the dopamine-coated surface, which is CNFs. The results showed that the higher the dopamine concentration, the larger the amount of deposited Pd, while the polymerization time is inversely proportional to the amount of Pd deposited. Thereby, the optimally functionalized surface for Pd deposition was found with a dopamine concentration of 3 mg mL−1 and a reaction time of 6 hours (PDAT1). This optimum Pd/CNF hybrid material showed very promising electrochemical and catalytic performances with a high discharge capacity of about 5.26 mA h cm−2 which could be maintained up to the 67th cycle at a cut-off capacity of 0.2 mA h cm−2 in non-aqueous Li–O2 batteries and an impressive catalytic activity for the oxygen reduction reaction via the preferred 4 electron pathway in aqueous electrolyte.
- This article is part of the themed collection: 2019 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...