CoII-catalyzed room-temperature growth of MnO2 on the skeleton of carbonized zeolitic imidazolate framework-67 crystals for boosting oxygen reduction reaction†
Abstract
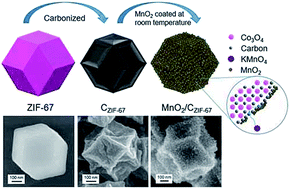
MnO2/C composites are widely used as cathode active materials or electrocatalysts in electrochemical power sources. Compared with a physical mixture of MnO2 and carbon materials, the growth of MnO2 on the surface of carbon can greatly improve the electrochemical performance of MnO2/C, and is usually performed at a temperature higher than 100 °C. From the viewpoint of sustainable development, the room-temperature growth of MnO2 on carbon would greatly reduce the cost and thermal pollution. Herein, at room temperature, we successfully planted MnO2 nanosheets over carbon particles, achieving MnO2/C composites through a redox reaction between MnO4− ions and carbonized zeolitic imidazolate framework-67 (ZIF-67) crystals (CZIF-67). Room-temperature growth of MnO2 may be attributed to the catalytic effect of CoII ions of Co3O4 nanoparticles formed during ZIF-67 carbonization. The resulting MnO2/CZIF-67 composites exhibit excellent catalytic performance towards the oxygen reduction reaction: the onset potential, the half-wave potential, and the electron transfer number are ∼0.88 V, ∼0.79 V and 3.6, respectively, which are close to the corresponding values of the state-of-the-art Pt/C. Also, the resulting MnO2/CZIF-67 shows better long-term durability and methanol tolerance than Pt/C, indicative of its potential applications in fuel cells and metal–air batteries.


 Please wait while we load your content...
Please wait while we load your content...