Mechanistic understanding of electrochemical plating and stripping of metal electrodes†
Abstract
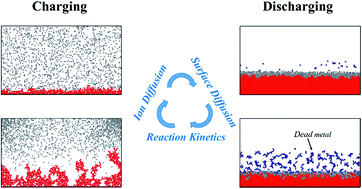
Lithium metal is an attractive negative electrode material for rechargeable lithium batteries because of its light weight and high electronegative redox potential. However, dendritic deposition of lithium during charging poses a safety concern. During discharging, some of the lithium may strip away from the electrode as the root of the dendrite is electrodissolved. This is referred to as dead lithium since it is not electrochemically active, which may result in low coulombic efficiency. In this work, a comprehensive understanding of the interface evolution leading to the formation of dead lithium is presented based on a mechanism-driven probabilistic analysis. A non-dendritic interface morphology is obtained under reaction and ionic transport controlled scenarios. Otherwise, this may evolve into mossy, dendritic, whisker or needle-like structures with the main characteristic being the propensity for undesirable vertical growth. During discharging, a pitted interface may be formed along with bulk dissolution. Surface diffusion is a key determinant controlling the extent of dead lithium formation, including a higher probability of the same when the effect of surface diffusion is comparable to that of ionic diffusion in the electrolyte and interface reaction.
- This article is part of the themed collection: 2019 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...
