Electrolyte regulation enhances the stability of Prussian blue analogues in aqueous Na-ion storage†
Abstract
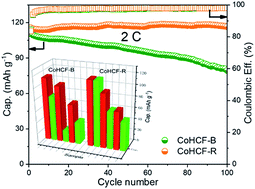
In this study, we employed Na2CoFe(CN)6 (CoHCF), which was synthesized by a commonly used co-precipitation method, as the cathode material for aqueous sodium-ion batteries (ASIBs). By adding an electrolyte additive (1 wt% CoSO4) in the aqueous electrolyte (1 M Na2SO4), the framework structures were stabilized and the cycle stability was significantly improved (100% capacity retention at a current density of 2C (1C = 120 mA g−1)) after 100 cycles without affecting the initial reversible capacity. Long-term cycle tests (2000 cycles) at current densities of 10C and 20C were also performed and indicated similar improvement. The XRD, FT-IR, and ICP-AES results demonstrated that better structure maintenance of the CoHCF framework can be realized when Co2+ ions were added. We suggest that such a simple method can be extended to all PBAs to improve the overall performance of SIBs effectively.


 Please wait while we load your content...
Please wait while we load your content...