Highly efficient and stable solid-state Li–O2 batteries using a perovskite solid electrolyte†
Abstract
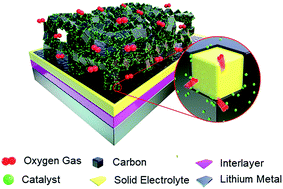
The solid-state Li–O2 battery is considered an ideal candidate for high-performance energy storage because of its high safety, due to use of non-flammable and non-volatile electrolytes, and high specific energy, as it uses Li metal and O2 gas as active materials. We present an original solid-state Li–O2 cell composed of a Li metal anode, a flexible polymer interlayer, a perovskite-structured Al-doped Li–La–Ti–O (A-LLTO) solid electrolyte, and an integrated cathode in which a porous A-LLTO solid electrolyte frame was covered with a carbon layer and CoO nanoparticles as the catalyst for the cyclic oxygen evolution and reduction reactions. The designed solid-state cell operated safely in pure O2 atmosphere at temperatures from 25 °C to 100 °C and delivered the first discharge capacity from 796 mA h gC+CoO−1 to 4035 mA h gC+CoO−1, respectively, at a current density of 0.05 mA cm−2. Notably, at 50 °C, the cell was maintained for 132 cycles under a limited capacity mode of 500 mA h gC+CoO−1 at a high current density of 0.3 mA cm−2, demonstrating the first step of success towards realizing Li batteries with high energy and cyclability, as well as safety.


 Please wait while we load your content...
Please wait while we load your content...