Kinetics of CO2 electrolysis on composite electrodes consisting of Cu and samaria-doped ceria†
Abstract
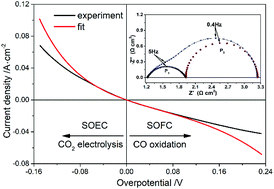
This work investigates the kinetics of CO2 electrolysis on Cu-SDC (samaria-doped ceria) composite electrodes supported on YSZ (yttria-stabilized zirconia) electrolytes. The Cu-SDC electrode was achieved by impregnating Cu into porous SDC with a copper nitrate solution. The electrochemical characteristics including polarization curves and AC impedance spectra were measured at different temperatures and applied biases, and in different atmospheres. The exchange current densities and charge transfer coefficients at various temperatures were obtained by applying nonlinear curve-fitting analysis to the current–overpotential curves. The polarization resistance derived from the I–V curve is consistent with that from the impedance spectrum, which indicates good reliability for fitting the Butler–Volmer equation to the I–V curve. The activation energy of CO2 electrolysis derived from the Arrhenius behavior of exchange current density is not affected by the atmosphere; however, it is decreased with the increase of impregnated Cu. The determined rate-limiting step is related to oxygen vacancies that play a vital role in CO2 electrolysis.


 Please wait while we load your content...
Please wait while we load your content...