Methane dry reforming catalyst prepared by the co-deflagration of high-nitrogen energetic complexes†
Abstract
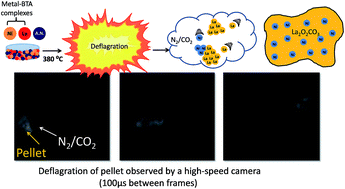
Methane dry reforming presents a unique opportunity to simultaneously consume both methane and carbon dioxide and generate from them clean-burning synthetic fuels for mobile energy applications. The industrialization of CH4 dry reforming has been impeded mainly due to problems relating to the catalyst–support interface including sintering and carbon accumulation. Here, we present a new methodology, termed co-deflagration, for the synthesis of a stable supported catalyst for methane dry reforming. Co-deflagration utilizes the energy released during the decomposition of high-nitrogen energetic ligands bound to a metal atom. During co-deflagration, the metal atoms crystallize into the supported catalyst material. Here, we used the co-deflagration technique to synthesize Ni/La2O2CO3 for use in methane dry reforming. We intended that the high thermal and kinetic energy imparted to La and Ni atoms during the energetic deflagration process would give rise to strong interfacial interaction between the catalyst and support and therefore stabilize catalytic activity. Ni/La2O2CO3 synthesized using co-deflagration showed stable performance under CH4 dry reforming conditions at elevated gas-hourly space velocities.


 Please wait while we load your content...
Please wait while we load your content...