Functionalization of the carbon additive of a high-voltage Li-ion cathode†
Abstract
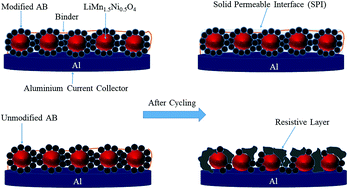
We report on the improved stability of high-voltage composite cathodes for lithium-ion batteries by chemical modification of the carbon additive, acetylene black, using diazonium chemistry with C6H4CF3, C6H4SO3H, C6H4COOH and C6H4N(C2H5)2 groups. Elemental analysis and X-ray photoelectron spectroscopy confirmed the presence of substituted aryl groups at the surface of the carbon. The electrochemical behavior of functionalized and unmodified carbon electrodes was investigated by cyclic voltammetry, galvanostatic cycling and electrochemical impedance spectroscopy. The irreversible capacity between 4.5 and 5.3 V vs. Li/Li+ strongly diminished after modification. The different functionalized carbons were utilized as a conductive additive in a high-voltage LiMn1.5Ni0.5O4 (LMN) cathode. Improved capacity retention was observed for LMN composite cathodes with modified carbons, as well as lower charge-transfer resistance determined after polarization for several hours at a constant voltage of 5.3 V. These observations were attributed to the presence of the grafted groups on the carbon additive, which inhibit the degradation of the electrolyte and the carbon, as demonstrated by X-ray photoelectron spectroscopy measurements of electrodes following charge/discharge cycling.


 Please wait while we load your content...
Please wait while we load your content...