Benzothiadiazole–ethynylthiophenezoic acid as an acceptor of photosensitizer for efficient organic dye-sensitized solar cells†
Abstract
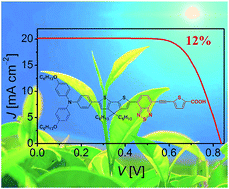
The logical linking of electron-rich and electron-deficient fragments has brought forth considerable advances in the power conversion efficiencies (PCEs) of organic dye-sensitized solar cells in the past decade. In this work, we construct a 5-(benzo[c][1,2,5]thiadiazol-4-ylethynyl)thiophene-2-carboxylic acid (BTETA) segment and employ it as the electron acceptor to exploit a donor–acceptor sensitizer in combination with the triarylamine-cyclopentadithiophene electron donor. With respect to its congener based on the efficient benzothiadiazole-benzoic acid (BTBA) acceptor, this employment of more electron-deficient acceptor BTETA does not alter the energy level of the highest occupied molecular orbital (HOMO) too much, but leads to an obviously downward displacement of the lowest unoccupied molecular orbital (LUMO) energy level. The resultant energy-gap shrinkage endows the new dye with a red-shifted absorption peak concomitant with a higher molar absorption coefficient, while this style of downward movement of the LUMO energy level does not exert a distinct impact on the electron injection efficiency from photoexcited dye molecules into titania, which endows the new dye with an enhanced photocurrent output, bringing forth an efficiency improvement up to 12.0% under 100 mW cm−2 simulated AM1.5 conditions.


 Please wait while we load your content...
Please wait while we load your content...