In situ derived Fe/N/S-codoped carbon nanotubes from ZIF-8 crystals as efficient electrocatalysts for the oxygen reduction reaction and zinc–air batteries†
Abstract
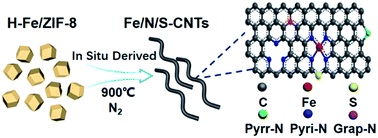
Here we develop for the first time Fe/N/S-codoped carbon nanotubes (Fe/N/S-CNTs), derived from hydrazine hydrate and ferrous sulfate treated metal–organic frameworks, as an efficient ORR catalyst in both alkaline and acidic electrolytes. Hydrazine hydrate serves as a reducing agent to prevent the rapid aggregation of Fe nanocatalysts, which facilitates the growth of CNTs during pyrolysis. And it is discovered for the first time that sulfate ions can be used as a sulfur source to create C–S–C bonds by reaction with carbon at a high temperature. The prepared Fe/N/S-CNTs exhibits excellent ORR activity with a half-wave potential of 0.887 V in alkaline medium, which is 42 mV higher than that of commercial Pt/C (0.845 V), and its half-wave potential is just 26 mV lower than that of Pt/C in acidic medium. In addition, it also has high stability and methanol resistance ability in both alkaline and acidic electrolytes. Furthermore, when used as the cathode in primary Zn–air batteries, the power density of Fe/N/S-CNTs reaches 111 mW cm−2, which is 1.5 times higher than that of Pt/C (73 mW cm−2). Experimental results and DFT calculations demonstrate that the high-yield of CNTs, the optimal balance ratio of pyridinic and graphitic N, and the synergistic effect of C–S–C and Fe–Nx are all essential ingredients for the outstanding ORR performance.


 Please wait while we load your content...
Please wait while we load your content...