Ni0.33Co0.67MoS4 nanosheets as a bifunctional electrolytic water catalyst for overall water splitting†
Abstract
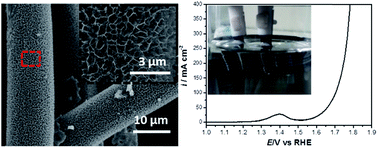
Currently, for overall water splitting, the highest activity for hydrogen evolution reaction (HER) oxygen evolution reaction (OER) are recorded by platinum (Pt) and ruthenium oxide (RuO2) catalysts, respectively. However, the shortage and sumptuosity of these noble metals limit their commercial applications. Herein, we prepared Ni0.33Co0.67MoS4 nanosheets on carbon fiber cloth (Ni0.33Co0.67MoS4/CFC) as bifunctional electrolytic water catalysts for both HER and OER. In the HER process, Ni0.33Co0.67MoS4/CFC exhibited an overpotential of only 48.0 mV at a current density of 10 mA cm−2, and the Tafel slope was 73.5 mV dec−1. Moreover, in the OER process, Ni0.33Co0.67MoS4/CFC displayed a low overpotential of 283 mV at current densities of 10 mA cm−2, and the Tafel slope was 68.8 mV dec−1. More importantly, Ni0.33Co0.67MoS4/CFC could act as a bifunctional electrocatalyst to split water under a cell voltage of 1.55 V at a current density of 10 mA cm−2. In addition, negligible decay occurred after 20 h of continuous electrolysis testing. Such noble-metal-free electrocatalysts may provide a strategy for the design of high efficiency overall water splitting catalysts, which are expected to be applied to the actual electrolysis water industry.


 Please wait while we load your content...
Please wait while we load your content...