3D nanoporous Ni/V2O3 hybrid nanoplate assemblies for highly efficient electrochemical hydrogen evolution†
Abstract
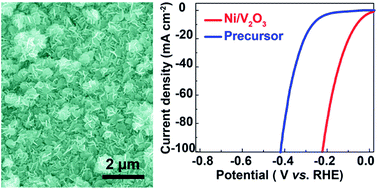
Nickel-based non-noble-metal materials have emerged as promising catalysts for electrochemical hydrogen production in view of their attractive intrinsic activities, electrical properties and low cost. Exploring new candidates for further improving the performances of nickel-based catalysts and understanding the structure–activity relationship are still necessary to reduce the overpotential of the hydrogen evolution reaction (HER) thus advancing their application in electrochemical water splitting. Herein, we developed a facile two-step self-templated strategy for fabricating a three-dimensional (3D) nanoporous nickel/vanadium oxide (Ni/V2O3) nanoplate assembly as a new efficient catalyst for alkaline HER. It is found that by controllably annealing the Ni–V–O assembly as a single precursor, Ni and V2O3 components are uniformly integrated in the nanoporous composite, showing a synergistically enhancing effect on the HER. The resulting 3D nanoporous structure not only creates numerous active sites accessible for the HER but also provides a conductive open network towards efficient electron/mass transport. Consequently, the nanoporous Ni/V2O3 nanoplate assembly exhibits excellent catalytic performance for alkaline HER in terms of a low overpotential of 61 mV at 10 mA cm−2 and a small Tafel slope of 79.7 mV dec−1 together with excellent long-term durability. These findings provide new insights into good design and construction of other highly active catalysts for diverse applications.


 Please wait while we load your content...
Please wait while we load your content...