Self-propagation high-temperature synthesis of half-Heusler thermoelectric materials: reaction mechanism and applicability†
Abstract
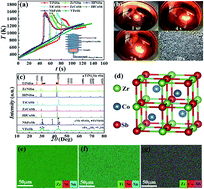
Combining excellent thermoelectric and mechanical properties, half-Heusler (HH) materials have been considered as one of the most promising candidates for thermoelectric applications in the high-temperature range. In this work, we apply the self-propagation high-temperature synthesis (SHS), a facile and scalable method, to HH thermoelectric materials. Comparing three families of HH materials, we show that MNiSn and MCoSb (M: Ti, Zr, Hf) can be successfully obtained by the SHS, but RFeSb (R: Nb, V) cannot. Our first-principles calculation suggests that the failure of SHS for RFeSb is because the formation enthalpy of these compounds is too low to overcome the reaction barrier. Our experiment also reveals a two-step reaction mechanism for the SHS of ternary HH compounds, i.e., two elements of lower melting points react first to form binary intermediate products and then the binaries react with the element of the highest melting point, where the second step contributes most of the heat released in the SHS, which is supported by our first-principles calculation. Finally, we optimize the HH materials through doping and show that the thermoelectric properties of SHS-synthesized HH compounds are comparable or even superior to those synthesized by laboratory-scale methods.


 Please wait while we load your content...
Please wait while we load your content...