Eutectic solvent-mediated selective synthesis of Cu–Sb–S-based nanocrystals: combined experimental and theoretical studies toward highly efficient water splitting†
Abstract
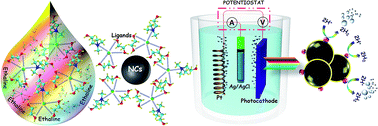
Recently, emerging Cu–Sb–S-based compounds have been identified as an attractive candidate for photovoltaic (PV) applications because of their high natural abundance, eco-friendly features and typical phase-dependent characteristics. Herein, a simple eutectic solvent-mediated (choline chloride/ethylene glycol) synthetic approach for newly debuted Cu–Sb–S-based nanocrystals (NCs) with phase-selective properties is presented. This combination of material and preparation method may promote the exchange of carriers by avoiding a steric hindrance for a facile charge transport encountered in NCs prepared using amines, thiols, hydrazines and phosphine oxide solvents. A temperature-dependent study of an ethaline-based deep eutectic solvent (DES) is conducted to elucidate the characteristics of associated chemical shifts and vibrations and to determine changes in hydrogen bonding interactions using structural and thermal analytical techniques. The results suggest that ethaline is a strong candidate as a greener solvent for the synthesis of NCs at relatively low temperatures. The electronic structures of all four Cu–Sb–S phases—Cu3SbS4, CuSbS2, Cu3SbS3, and Cu12Sb4S13—were simulated using the Vienna ab initio Simulation (VASP) code, projector augmented-wave (PAW) potentials and the hybrid functional method (HSE 06) and using density functional theory for combined theoretical and experimental studies. Discrepancies between the experimental and theoretical bandgap values of 0.29, 0.18, 0.12 and 0.16 eV were observed for Cu3SbS4, CuSbS2, Cu3SbS3 and Cu12Sb4S13 compounds, respectively. A photoelectrochemical (PEC) water reduction system with a Mo/photoelectrode/CdS/Pt/electrolyte configuration generated a cathodic photocurrent of −1.28 and −2.72 mA cm−2 for Cu3SbS4 and CuSbS2 electrodes, respectively, at 0 V versus the reversible hydrogen electrode (VRHE) under AM 1.5 G illumination, demonstrating the great potential of NCs prepared via eutectic solvent-mediated synthesis. This is the first successful attempt to apply eutectic solvent-mediated Cu–Sb–S NCs for solar driven H2 production. These outcomes suggest that designing proper functional materials through the application of greener synthesis strategies can improve water-splitting performance and would help meet the perpetual technological need for greener methods.


 Please wait while we load your content...
Please wait while we load your content...