Shape- and size-controlled synthesis of Cu nanoparticles wrapped on RGO nanosheet catalyst and their outstanding stability and catalytic performance in the hydrogenation reaction of dimethyl oxalate†
Abstract
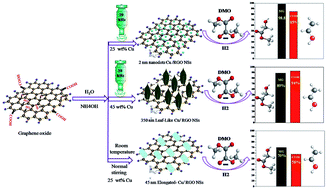
Considerable interest has been paid in the last few years to the important environmentally benign catalytic hydrogenation reaction of dimethyl oxalate (DMO) to produce methyl glycolate (MG), ethylene glycol (EG) and ethanol (ETOH). SiO2-supported noble metal elements, such as Au, Ag and Ru, have been nominated as efficient catalysts for the production of high MG selectivity. In this work, noble-metal-free Cu nanoparticles wrapped on reduced graphene oxide (RGO) nanosheets were synthesized using a green in situ sonochemical method in an aqueous medium and then applied without the calcination step as a robust catalyst in the DMO hydrogenation reaction. Moreover, the effects of ultrasound (US), ammonia (NH4OH), a combination of both US and NH4OH, and Cu loading (10, 25 and 45 wt%) on the morphology and catalytic performance of the produced Cu/RGO catalysts were studied in detail. Interestingly, transmission electron microscopy images revealed that a dramatic morphology evolution of the Cu catalysts from ultra small dots to elongated and leaf-like nanoparticles shapes occurred, depending on simple modification of the reaction parameters. Notably, the obtained catalytic results demonstrated that among the different produced six catalysts, the prepared Cu/RGO catalyst using 20 kHz of ultrasound and in the presence of ammonia with 25 wt% Cu loading displayed the highest MG selectivity (98.8%) at a relatively low reaction temperature (210 °C), while, the synthesized Cu/RGO catalyst with 45 wt% Cu loading exhibited the highest ETOH selectivity (94%) at 240 °C. Moreover, both catalysts showed long-term stability for at least 300 h while maintaining the high DMO conversion and selectivity ratio. The comprehensive characterization to the as-prepared, reduced and spent catalysts concluded that the synergistic ratio of Cu+/(Cu+ + Cu0), the high dispersion of Cu nanoparticles, the defective edges, sites and electron transfer provided from RGO were the main reasons for the obtained superior catalytic activity and long-term stability.


 Please wait while we load your content...
Please wait while we load your content...