Ultrafast solid-state lithium ion conductor through alloying induced lattice softening of Li6PS5Cl†
Abstract
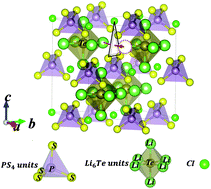
The development of a solid electrolyte with superb Li+ conductivity is the key to enabling safe and high-performance all-solid lithium ion batteries which are free of the safety issues associated with flammable organic liquid electrolyte. Systematic experimental work has been carried out as a follow up to our recent theoretical prediction that superb ionic conductivity could be achieved through the tuning of the lattice chemistry in the cubic argyrodite Li6PS5Cl. Remarkable enhancement of the ionic conductivity has been achieved through the combined effect of excess Li and substitution of S with Te. This leads to an alloy, Li6.25PTe0.125S5.125Cl0.75, having the superb Li+ conductivity of 4.5 mS cm−1 at 25 °C, together with a low activation energy of 0.16 eV to safeguard adequate ionic conductivity at low temperature. This current work conclusively affirms the theoretical outcome that the superb ionic conductivity is attributed to weakened bonding, an enlarged unit cell, and a softened lattice, making it easier for the long-distance diffusion of Li+. In addition, this new class of solid electrolyte based on cubic chalcogenide is electrochemically compatible with the Li anode over a large potential window up to 7 V, which is highly desirable for high performance solid lithium ion batteries.


 Please wait while we load your content...
Please wait while we load your content...