Synthesis, crystal structure, and ionic conductivity of hydride ion-conducting Ln2LiHO3 (Ln = La, Pr, Nd) oxyhydrides†
Abstract
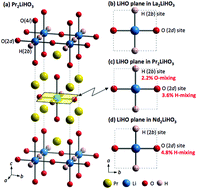
Hydride ion (H−)-conducting solids could contribute to the development of novel electrochemical devices using unique H− conduction phenomena. However, understanding the new class of H− conducting oxyhydrides is still undergoing. In this study, new H− conducting Ln2LiHO3 (Ln = La, Pr, Nd) oxyhydrides were synthesised using a high-pressure (2 GPa) method. Structural analysis revealed that the anion arrangement in the axial sites of the perovskite unit depends on the Ln ions and strongly affects hydride conductivity. Nd2LiHO3, which has a space group Immm, in which ∼5% of the O sites (2d) are occupied instead by H, had the highest conductivity at 280 °C of 2.2 × 10−5 S cm−1. The possibility of long-distance H− diffusion in Ln2LiHO3 was computationally investigated. The results corroborate the experimental findings, which indicate that lower O content promotes anion migration.


 Please wait while we load your content...
Please wait while we load your content...