Redox inactive ion meliorated BaCo0.4Fe0.4Zr0.1Y0.1O3−δ perovskite oxides as efficient electrocatalysts for the oxygen evolution reaction†
Abstract
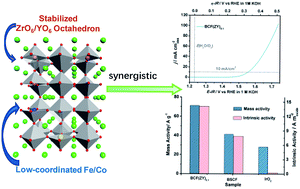
Exploring competent electrocatalysts for the oxygen evolution reaction (OER) is central to the development of a clean, reliable and emission-free hydrogen economy. Perovskites have been seen as promising OER electrocatalysts among all the potential candidates so far. Here, we introduced redox inactive Zr and Y ions to meliorate the redox capability of active ions and prepared BaCo0.5−xFe0.5−xZrxYxO3−δ (BCF(ZY)x, x = 0.0−0.2) oxides as efficient electrocatalysts for the OER. The catalyst of BCF(ZY)0.1 exhibits a higher OER activity (including disk activity, intrinsic activity and mass activity) and better durability than the gold-standard OER electrocatalyst IrO2 and the well-known Ba0.5Sr0.5Co0.8Fe0.2O3−δ (BSCF) perovskite. The overpotential to deliver an electrode current density of 10 mA cm−2 of BCF(ZY)0.1 oxide is merely 0.32 V in 1 M KOH, and the intrinsic activity of BCF(ZY)0.1 is ∼52 times higher than IrO2. The outstanding electro-catalytic activity and stability of BCF(ZY)0.1 are attributed to the increased redox ability compared with BSCF, including the faster charge transfer process and much more facile adsorption of OH− and desorption of O2.


 Please wait while we load your content...
Please wait while we load your content...