Vanadium oxide nanoparticles supported on cubic carbon nanoboxes as highly active catalyst precursors for hydrogen storage in MgH2†
Abstract
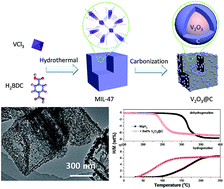
Magnesium hydride (MgH2) has attracted intense interest as a high-capacity hydrogen storage material. However, high thermal stability and slow kinetics limit its practical applications. Herein, vanadium oxide nanoparticles supported on cubic carbon nanoboxes (nano-V2O3@C) are synthesized successfully by using MIL-47(V) as a precursor, and superior catalytic effects derived from the nano-V2O3@C composite towards the hydrogen storage reaction of MgH2 are demonstrated. The MgH2-9 wt% nano-V2O3@C sample starts releasing hydrogen at 215 °C, which is 60 °C lower than that of the additive-free MgH2. At 275 °C, approximately 6.4 wt% of hydrogen is released from the MgH2-9 wt% V2O3@C sample within 20 min. The dehydrogenated sample absorbs hydrogen even at room temperature under 50 bar of hydrogen pressure, and rehydrogenation is complete within 700 s at 150 °C. XRD and XPS measurements identify the existence of metallic V after ball milling, and its presence remains nearly constant in the subsequent dehydrogenation/hydrogenation process upon heating. Further ab initio calculations reveal that the presence of V facilitates the breaking of the Mg–H bond of the MgH2 unit, which is reasonably responsible for the significantly reduced operating temperatures and improved kinetics of the V-catalysed MgH2.


 Please wait while we load your content...
Please wait while we load your content...