A comparative study on nanocrystalline layered and crystalline cubic TiP2O7 for rechargeable Li/Na/K alkali metal batteries†
Abstract
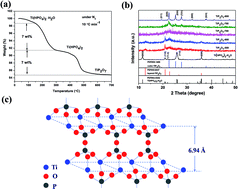
We report, for the first time, that a nanocrystalline layered TiP2O7 as a cathode material for Li-, Na- and K-alkali metal batteries is prepared by a solvothermal process followed by a heat treatment at 600 °C. For comparison, a crystalline cubic TiP2O7 is also synthesized at 800 °C. It is clarified that the layered TiP2O7 is slightly poorer for lithium storage but much better for sodium and potassium storages than the cubic phase. The detailed electrochemical processes are investigated by means of ex situ XRD analyses. Different from the two-phase co-existence mechanism for the cubic phase, a solid-solution mechanism is identified for the layered phase to follow in the Li/Na/K intercalation reactions based on electrochemical and structural studies. To overcome the poor electronic conductivity limit for high rate applications, carbon coating is employed on the TiP2O7 samples. The carbon-coated layered phase TiP2O7 (600 °C) displays fairly reversible capacity close to 100 mA h g−1 in both Li and Na rechargeable alkali metal batteries. It is a promising electrode material for large energy storage systems.


 Please wait while we load your content...
Please wait while we load your content...