Enhanced polysulfide redox kinetics electro-catalyzed by cobalt phthalocyanine for advanced lithium–sulfur batteries†
Abstract
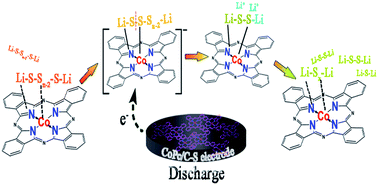
Controlling the intrinsic polysulfide shuttle is the key to enhance the reaction kinetics and cycling stability of lithium–sulfur batteries. Herein, active transition-metal phthalocyanine compounds are firstly adopted as the electrocatalysts for sulfur cathodes and proved to enhance the cycling stability. Systematic investigations specifically for cobalt phthalocyanine (CoPc) suggest that the inclusion of CoPc can inhibit the self-discharge behavior and improve the exchange current density of sulfur cathodes effectively. Benefiting from the strong adsorption capability toward soluble polysulfide species, favorable guidance for the formation of Li2S2/Li2S and efficient catalytic transformation of the deposits in the subsequent charging process, the sulfur electrodes containing 3.8 wt% CoPc deliver a specific capacity of 719.6 mA h g−1 after 400 cycles at 0.2C, 80% higher than those of the pristine ones. A comparative study on CoPc, CoS, cobalt- and Pc-containing compounds confirms that the effectiveness of the electrocatalyst is closely related to the integrated Co–N4 unit and a probable catalytic mechanism is proposed accordingly.


 Please wait while we load your content...
Please wait while we load your content...