Anthraquinone thin-film electrodes for reversible CO2 capture and release†
Abstract
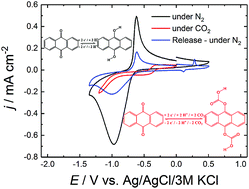
We report reversible electrochemical capture and release of carbon dioxide using the well-known dye precursor and industrial catalyst anthraquinone. Although quinones are well-studied for electrochemical capture and release of CO2 in solution, we have discovered that a 100 nm film of anthraquinone can realize this in a heterogeneous fashion. In-depth spectroelectrochemical studies were performed in order to study the mechanism of this CO2 capture and release. Anthraquinone films reached an uptake capacity of 5.9 mmolCO2 gAQ−1.


 Please wait while we load your content...
Please wait while we load your content...