A novel solvent-free strategy for the synthesis of bismuth oxyhalides†
Abstract
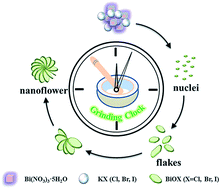
As an important photocatalytic semiconductor, bismuth oxyhalides (BiOX, X = Cl, Br, and I) are typically prepared by liquid-phase strategies in the presence of solvents, which possess potential environmental implications. Herein, we demonstrate a fast and simple solvent-free mechanical grinding method for eco-friendly synthesis of BiOX for the first time. By mixing and grinding bismuth nitrate pentahydrate with potassium halides, BiOCl, BiOBr, and BiOI hierarchical flower-like nanostructures can be successfully prepared within 5 min. The possible reaction mechanism and growth process of the crystals are proposed based on the experimental results. Photoelectric conversion and coupling adsorption–photodecomposition for wastewater purification both confirm that the as-prepared BiOX (X = Cl, Br, and I) possess favorable and stable photocatalytic activities. Consequently, the high efficiency solvent-free mechanical grinding method can be further investigated as a convenient method for economic industrial-scale preparation of bismuth oxyhalide composites with excellent photocatalytic activity.


 Please wait while we load your content...
Please wait while we load your content...