Maximising the hydrogen evolution activity in organic photocatalysts by co-polymerisation†
Abstract
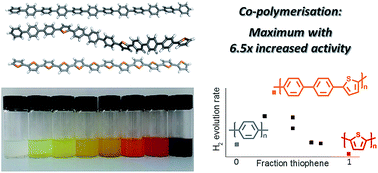
The hydrogen evolution activity of a polymeric photocatalyst was maximised by co-polymerisation, using both experimental and computational screening, for a family of 1,4-phenylene/2,5-thiophene co-polymers. The photocatalytic activity is the product of multiple material properties that are affected in different ways by the polymer composition and microstructure. For the first time, the photocatalytic activity was shown to be a function of the arrangement of the building blocks in the polymer chain as well as the overall composition. The maximum in hydrogen evolution for the co-polymer series appears to result from a trade-off between the fraction of light absorbed and the thermodynamic driving force for proton reduction and sacrificial electron donor oxidation, with the co-polymer of p-terphenyl and 2,5-thiophene showing the highest activity.


 Please wait while we load your content...
Please wait while we load your content...