Boosting the oxygen reduction activity of a three-dimensional network Co–N–C electrocatalyst via space-confined control of nitrogen-doping efficiency and the molecular-level coordination effect†
Abstract
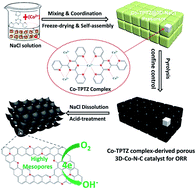
The improvement of total nitrogen content and nitrogen-doping efficiency in carbon-based electrocatalysts is greatly significant to boost the electrocatalytic activity for the oxygen reduction reaction (ORR). Here, we report a new strategy for the synthesis of a highly mesoporous cobalt and nitrogen co-doped carbon electrocatalyst (3D-Co–N–C) with a three-dimensional network structure and a high BET surface area (∼638 m2 g−1) via using a novel cobalt-2,4,6-tri(2-pyridyl)-1,3,5-triazine complex with a strong molecular-level coordination effect as a single-source precursor and self-assembled sodium chloride aggregates as a space-confined nanoreactor for effective control of a high-temperature calcination process to reduce the thermal loss of nitrogen atoms and promote the nitrogen-doping efficiency, facilitating boosting of the ORR electrocatalytic activity in alkaline medium. The prepared 3D-Co–N–C catalyst exhibits unexpectedly excellent ORR activity with an onset potential of ∼1.0 V and a half-wave potential of ∼0.83 V, which is comparable to that of the commercial 20 wt% Pt/C catalyst. Additionally, the H2O2 yield (<17.0%) and the average electron transfer number of ∼3.8 for 3D-Co–N–C indicate a quasi four-electron pathway for ORR catalysis, suggesting that 3D-Co–N–C is a promising carbon-based electrocatalyst. It is proposed that the formation of the Co–Nx active structure can effectively enhance the electrocatalytic activity, but high contents of pyridinic- and graphitic-N can be mainly responsible for the ORR activity, which may be the electrocatalytically active site centers for the ORR. Besides, high BET surface area, highly mesoporous characteristics and outstanding electronic conductivity are also significant for the improvement of ORR activity. This study can provide a new, facile and green method for building high-performance carbon-based ORR electrocatalysts derived from easily available and innoxious transition metal–organic complexes, which can also help us to better understand the origin of the activity, active sites and their catalysis mechanism.


 Please wait while we load your content...
Please wait while we load your content...