The roles of oxygen vacancies, electrolyte composition, lattice structure, and doping density on the electrochemical reactivity of Magnéli phase TiO2 anodes†
Abstract
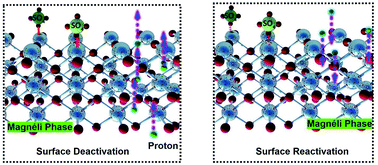
Substoichiometric TiO2 (TinO2n−1, 4 ≤ n ≤ 10) is a promising and cost-effective material, that is being investigated for many applications, such as information storage, energy storage and conversion, and water treatment. Upon extended anodic polarization, TinO2n−1 reportedly suffers from gradual loss in conductivity and electrochemical reactivity. In this study, the surface deactivation and reactivation mechanisms were examined on a TinO2n−1 monolithic electrode in three different electrolyte solutions (i.e., H2SO4, HClO4, HCl). The intrinsic electronic properties, charge transfer kinetics, crystalline structure, and surface composition were examined experimentally after anodic and cathodic polarizations. Statistically equivalent results were obtained from local scanning electrochemical microscopy (SECM) and bulk electrochemical impedance spectroscopy measurement, which indicate that spatially resolved SECM data accurately characterized charge transfer kinetics of the TinO2n−1 electrode at the micron-scale. Results indicate that decreases in conductivity and charge transfer kinetics after anodic polarizations in all three electrolytes were primarily attributed to the loss of charge carriers, such as H+ discharge at Ti3+ point donor sites, and the process was reversible during cathodic polarization via H+ intercalation. In the H2SO4 electrolyte reversible surface passivation also occurred, which was attributed to the formation of TiOSO4 surface species whose presence were supported by experimental measurements and density functional theory calculations. It was also determined that the TinO2n−1 crystal structure directly affected the hydroxyl radical formation rate, with the highest rate observed for Ti4O7, which also possessed the highest charge carrier density.


 Please wait while we load your content...
Please wait while we load your content...
