Highly active and stable electrocatalyst of Ni2P nanoparticles supported on 3D ordered macro-/mesoporous Co–N-doped carbon for acidic hydrogen evolution reaction†
Abstract
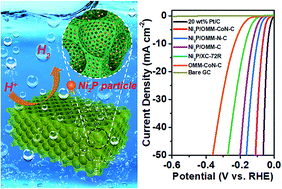
The development of highly efficient and stable non-precious metal electrocatalysts to substitute Pt for the hydrogen evolution reaction (HER) is significant for hydrogen-based energy technologies. Herein, we fabricated Ni2P nanoparticles supported on 3D ordered macro-/mesoporous Co–N-doped carbon (Ni2P/OMM-CoN-C) and applied it as a robust HER electrocatalyst under strong acidic conditions. In the as-prepared Ni2P/OMM-CoN-C, ultrafine Ni2P nanoparticles with size of 3–5 nm are evenly distributed in the mesopore walls of the carbon matrix. The Ni2P/OMM-CoN-C catalyst exhibits excellent HER electrocatalytic performance with distinctly low overpotential of 68 mV at 10 mA cm−2, small Tafel slope of 37 mV dec−1, and excellent long-term stability, rendering it as a very promising substitute for the Pt catalyst. The superior catalytic performance of Ni2P/OMM-CoN-C could be ascribed to the synergetic effect of the highly dispersed active Ni2P nanoparticles and the porous Co–N-doped carbon support with 3D hierarchical architecture consisting of ordered interconnected macropores and mesopores, which offers the distinct advantages of highly exposed active sites, high-efficiency mass transport, and great electronic conductivity.


 Please wait while we load your content...
Please wait while we load your content...