Sulfur: an intermediate template for advanced silicon anode architectures†
Abstract
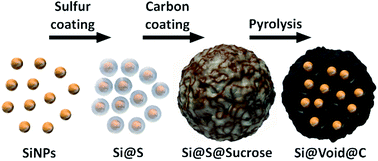
The lithium–sulfur chemistry provides a next generation battery technology on the verge of commercialization with significantly increased specific energy. However, the cycle life mainly suffers from dendrite and continuous SEI formation in lithium anodes inducing active material and electrolyte depletion. Here, we report on a silicon–carbon composite anode as a stable alternative anode for safe Li–S cells. Well-defined sulfur coatings generate a shell for a silicon core (Si@S) to further form a carbon shell (Si@S@sucrose). After sulfur removal the void structure (Si@void@C) allows to compensate the mechanical stress imposed by the huge volume change during the lithiation of the silicon. In this case, sulfur is not only used as a low cost and high capacity cathode material but also as a template to create free volume. It is easily removed during the pyrolysis and no acid leaching steps are required. In half cell tests vs. lithium a high capacity of 2270 mA h gSi−1 (690 mA h g−1) was achieved in the 10th cycle and the reversible lithiation of the silicon particles could be ensured for more than 50 cycles. The prelithiated Si–C anode with a high areal capacity of 2 mA h cm−2 was successfully matched with a sulfur cathode in a SLS full cell on coin cell and on pouch cell levels. A high capacity of about 807 mA h gsulfur−1 (2nd cycle) was reached with a low lithium excess of only 76% compared to 2000% lithium excess in state-of-the-art Li–S cells.


 Please wait while we load your content...
Please wait while we load your content...