Investigation of n-type doping strategies for Mg3Sb2†
Abstract
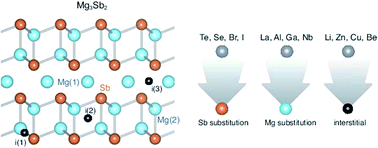
Recent, and somewhat surprising, successful n-type doping of Mg3Sb2 was the key to realizing high thermoelectric performance in this material. Herein, we use first-principles defect calculations to investigate different extrinsic n-type doping strategies for Mg3Sb2 and to reveal general chemical trends in terms of dopant solubilities and maximal achievable electron concentrations. In agreement with experiments, we find that Sb substitution is an effective doping strategy, with Se and Te doping predicted to yield up to ∼8 × 1019 cm−3 electrons. However, we also find that Mg substitution with trivalent (or higher) cations can be even more effective; in particular, the predicted highest achievable electron concentration (∼5 × 1020 cm−3) with La as an extrinsic dopant exceeds that of Se and Te doping. Interstitial doping (Li, Zn, Cu, Be) is found to be largely ineffective either due to self-compensation (Li) or high formation energy (Zn, Cu, Be). Our results offer La as an alternative dopant to Te and Se and reinforce the need for careful phase boundary mapping in achieving high electron concentrations in Mg3Sb2.


 Please wait while we load your content...
Please wait while we load your content...
