FeOx/FeP hybrid nanorods neutral hydrogen evolution electrocatalysis: insight into interface†
Abstract
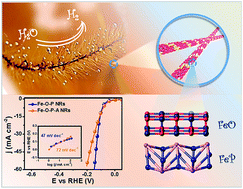
The electrocatalytic interface plays a crucial role in driving the water splitting reaction. Herein, we report a rational constructed interface of Fe–O–P hybrid nanorods co-catalyst playing a significant role in high-performance hydrogen generation from neutral water. It is worth noting that the FeOx/FeP hybrid structure exhibits remarkably low overpotential of 96 mV at a current density of 10 mA cm−2 with a small Tafel slope of 47 mV dec−1 and maintains excellent electrolytic durability over 60 h, placing it at the forefront among the best hydrogen evolution electrocatalysts operating in neutral media. The increased Tafel value contributed by FeOx elimination demonstrates the important effect of the FeOx/FeP interface. Additionally, theoretical investigations reveal deeper insights into the FeOx/FeP interface: firstly, the FeOx facilitates the adsorption and dissociation of water molecules and accelerates the supply of hydrogen atoms in the Volmer–Heyrovsky reaction; secondly, the interface further optimizes the Gibbs free energy for hydrogen adsorption at the FeP surface.


 Please wait while we load your content...
Please wait while we load your content...