A flexible, self-healing and highly stretchable polymer electrolyte via quadruple hydrogen bonding for lithium-ion batteries†
Abstract
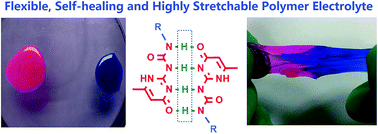
Polymer electrolytes are envisioned to be promising alternatives to their liquid counterparts in lithium-ion batteries as they are able to address electrolyte inflammability and leakage issues. However, polymer electrolytes usually suffer from cracks or breakage, which could lead to short circuits and bring severe safety issues. Herein, we propose a paradigm to address these problems in which the polymer electrolyte is formed by a physically cross-linked network via ureidopyrimidinone (UPy) containing brush-like poly(ethylene glycol) chains. The formed novel polymer electrolyte is flexible and able to provide fast intrinsic self-healability and high stretchability. It can heal the cut damage within 2 hours under ambient conditions without any external stimulus and can be stretched to more than 20 times its length without breaking. Furthermore, the novel polymer electrolyte exhibits excellent interfacial stability with electrodes, resulting in high performance batteries with stable reversible electrochemical reaction and cycling.


 Please wait while we load your content...
Please wait while we load your content...