Ag3V2(PO4)2F3, a new compound obtained by Ag+/Na+ ion exchange into the Na3V2(PO4)2F3 framework†
Abstract
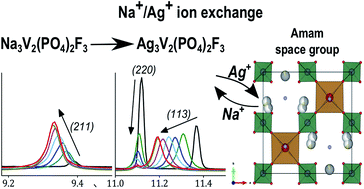
Phosphate polyanionic compounds have been used for several technological applications and are especially widespread in battery research. Na3V2(PO4)2F3 is a material that holds great promise as a positive electrode for Na-ion batteries. We study here the Ag+/Na+ ion exchange that is possible due to the high ionic mobility of Na+ in this material. A nearly complete ion exchange was obtained and we report the crystal structure of the new orthorhombic phase Ag3V2(PO4)2F3, determined by synchrotron X-ray and neutron powder diffraction. Silver occupies the same crystallographic sites as sodium and, except for the differences caused by steric effects, Ag3V2(PO4)2F3 preserves the symmetry constraints already present in the parent compound Na3V2(PO4)2F3. We also followed the evolution of the crystal structure upon heating/cooling, to observe an order/disorder transition analogous to the one already reported for Na3V2(PO4)2F3, but occurring at a significantly higher temperature.


 Please wait while we load your content...
Please wait while we load your content...