Significantly optimized thermoelectric properties in high-symmetry cubic Cu7PSe6 compounds via entropy engineering
Abstract
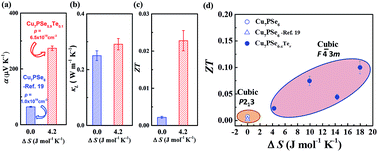
High-symmetry crystal structures are preferred for thermoelectrics because high structural symmetry usually yields good electron transport properties. Entropy engineering is an effective approach to improve the structural symmetry of low-symmetry materials, and thus to enhance their thermoelectric performance. In this study, via introducing Te into the argyrodite-type compound Cu7PSe6, the configurational entropy is significantly increased to successfully improve its initial low-symmetry cubic structure (P213) to the high-symmetry cubic structure (F![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m) at room temperature. Such improved structural symmetry leads to a high density-of-state effective mass but similar carrier mobility in the same carrier concentration range as compared with the pristine Cu7PSe6. Thus, significantly optimized electron transport properties are achieved in the Te-alloyed Cu7PSe6 samples. In particular, at room temperature, the power factor of the high-symmetry cubic Cu7PSe5.7Te0.3 sample is about 15-times higher than that of the low-symmetry Cu7PSe6 matrix. Combining the well-maintained ultralow lattice thermal conductivity, a maximum ZT of around 0.55 at 600 K is obtained in Cu7PSe5.7Te0.3. This work strongly shows that entropy engineering using multiple components is a very powerful strategy to discover or design novel high-performance TE materials starting from low-symmetry compounds.
3m) at room temperature. Such improved structural symmetry leads to a high density-of-state effective mass but similar carrier mobility in the same carrier concentration range as compared with the pristine Cu7PSe6. Thus, significantly optimized electron transport properties are achieved in the Te-alloyed Cu7PSe6 samples. In particular, at room temperature, the power factor of the high-symmetry cubic Cu7PSe5.7Te0.3 sample is about 15-times higher than that of the low-symmetry Cu7PSe6 matrix. Combining the well-maintained ultralow lattice thermal conductivity, a maximum ZT of around 0.55 at 600 K is obtained in Cu7PSe5.7Te0.3. This work strongly shows that entropy engineering using multiple components is a very powerful strategy to discover or design novel high-performance TE materials starting from low-symmetry compounds.


 Please wait while we load your content...
Please wait while we load your content...