Ultrasensitive and selective electrochemical sensing of Hg(ii) ions in normal and sea water using solvent exfoliated MoS2: affinity matters†
Abstract
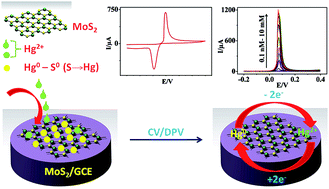
In this work, solvent exfoliated molybdenum disulphide (MoS2) has been exploited for the electrochemical (EC) sensing of mercury(II) ions (Hg2+). A 30-fold increase in peak current compared to that of a glassy carbon electrode (GCE) and a detection limit (LOD) of 0.000001 nM, i.e. in parts per quadrillion (0.2 ppq) levels, which satisfactorily meets the sensitivity requirement of the U.S. Environmental Protection Agency (EPA), were exhibited by the MoS2/GCE. Furthermore, the MoS2 electrode demonstrated an excellent selectivity towards Hg2+ with almost nil current response for most other metal ions except Ag+. No interference was observed from Ag+ during simultaneous determination of Hg2+ and Ag+ as the latter peak was observed at a higher peak potential with a ΔE of ∼0.4 V. The MoS2/GCE extended the remarkable sensing of Hg2+ to real water samples such as tap and sea water. The sensitivity of MoS2 for Hg2+ was found to be superior than that of reduced graphene oxide (rGO) and graphene oxide (GO) (∼4 & 30 times, respectively). The superior sensing of Hg2+ by MoS2 was assigned to the synergy of the affinity (between the Hg2+ and S2− groups) and the semiconducting properties of MoS2. It is notable that this performance of MoS2 is without any modifications except for simple solvent ultrasonication.


 Please wait while we load your content...
Please wait while we load your content...