Irreversible water mediated transformation of BCN from a 3D highly porous form to its nonporous hydrolyzed counterpart†
Abstract
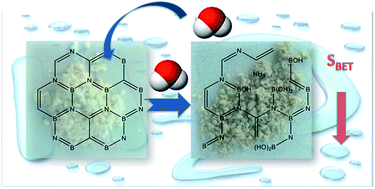
Boron carbon nitrides (BCNs) were synthesized from boric acid and melamine mixtures of various ratios, by heat treatment at 1000 °C. They are thermally stable nanorods of a high carbon content (∼20 at%) and low crystallinity. The various precursor ratios led to alterations in porosity and in B, C, and N chemical environments. Since the applications of this kind of material in aqueous media are of high interest, their stability upon exposure to water was investigated. Adsorption of water resulted in a marked weight gain and in a loss of porosity and fluorescence. FTIR and XPS analyses indicated that contact with moisture/water resulted in the formation of ammonium borate and B–C bonds were identified as the main sites of this reaction. BCN reacts slowly with air humidity. In this process ammonia is released and the adsorptive properties are gradually lost. The results suggest that BCN when used in an aqueous phase/ambient environment should be considered as rather nonporous BCNO with a high content of a carbon phase (C–C), significant amount of borate, and lower nitrogen content than those in freshly synthesized samples.


 Please wait while we load your content...
Please wait while we load your content...