Defect chemistry and electrical properties of sodium bismuth titanate perovskite
Abstract
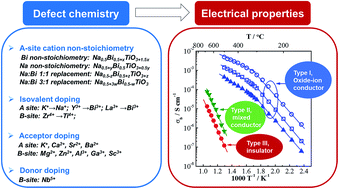
The ferroelectric perovskite Na0.5Bi0.5TiO3, NBT, can exhibit three types of electrical behaviour, i.e. oxide-ion conduction (type I), mixed ionic–electronic conduction (type II) and insulating/dielectric (type III) based on various defect mechanisms. Here we review how to tune the electrical properties of NBT via several mechanisms, including A-site Na or Bi non-stoichiometry, isovalent substitution, and acceptor- and donor-doping. The diversity of the electrical behaviour in the NBT lattice is attributed to the high level of oxide-ion conductivity originating from highly mobile oxygen ions which can be fine-tuned to optimise or suppress ionic conduction. High oxide-ion conductivity can be obtained by manipulating the starting Na/Bi ≥1 and by acceptor-doping to make NBT a potential electrolyte material for intermediate temperature solid oxide fuel cells (IT-SOFCs). In contrast, the oxide-ion conduction can be partially or fully suppressed by having a starting (nominal) composition with Na/Bi <1, donor-doping, or utilising the trapping effect between oxygen vacancies and some B-site acceptor dopants. This significantly reduces the dielectric loss and makes NBT-based materials excellent candidates as high-temperature dielectrics for capacitor applications.
- This article is part of the themed collections: Recent Review Articles, Celebrating our 2019 Prize and Award winners, Celebrating Excellence in Research: Women of Materials Science and Advances in Solid State Chemistry and its Applications


 Please wait while we load your content...
Please wait while we load your content...