On the chemistry and electrochemistry of LiPON breakdown†
Abstract
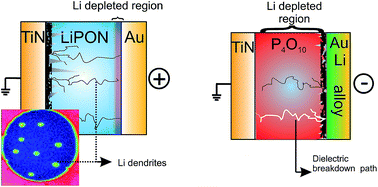
Battery safety and cycle life remain of concern to current Li-ion batteries that use a liquid organic electrolyte. Both issues can be resolved by employing a solid electrolyte. Numerous Li-ion conducting solids are known today, however the stability of most of these is too low to engender widespread usage. Here, we report on the decomposition chemistry and electrochemistry of LiPON, at present the most commonly used solid electrolyte. The decomposition potential was calculated from basic thermodynamic quantities and was linked to current–voltage (I–V) measurements with great consistency. The decomposition of LiPON was shown to occur at a potential of 4.3 V and proceeds in a diffusion limited way. The LiPON layer decomposes into Li-ions, O2 or N2 gas and a phosphate rich compound. Ultimately hard breakdown could occur through the formation of metallic lithium filaments that short circuit the LiPON layer as shown by TOF-SIMS imaging experiments. As such the work provided here also provides insight in the breakdown mechanism of RRAM devices.


 Please wait while we load your content...
Please wait while we load your content...