Iron-assisted engineering of molybdenum phosphide nanowires on carbon cloth for efficient hydrogen evolution in a wide pH range†
Abstract
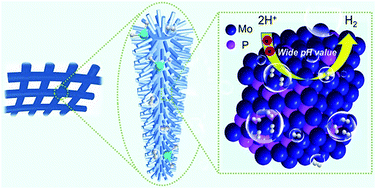
Exploration of active, cost-effective, and stable non-noble electrocatalysts for efficient hydrogen evolution is essential for sustainable energy systems. In this study, we report the synthesis of molybdenum phosphide nanowires on carbon cloth (MoP NW/CC) by a facile iron-assisted strategy; the as-obtained MoP NW/CC is a robust hydrogen evolution catalyst with high activity. Results reveal that the added iron can promote the upward growth of ferrum molybdenum oxide (Fe–Mo–O) nanowire precursor on carbon cloth and morphological maintenance during the in situ gas–solid phosphidation process; thus, iron plays a key role in engineering the morphology of the catalysts. As a novel 3D hydrogen evolution cathode, the as-obtained MoP NW/CC electrode exhibits a low onset potential of 72 mV, low overpotential of 173 mV for high current density of 100 mA cm−2, a small Tafel slope of 53.3 mV dec−1, and superior durability in an acidic electrolyte. Additionally, this electrode displays considerable HER activity and promising stability in alkaline and neutral media. This outstanding HER activity for MoP NW/CC can be ascribed to its unique nanowire morphology with a large surface area exposing ample active sites and superior charge transport kinetics. Importantly, in our study, a facile and effective approach has been developed for regulating the morphology of transition-metal phosphides.


 Please wait while we load your content...
Please wait while we load your content...