High capacity V-based metal hydride electrodes for rechargeable batteries†
Abstract
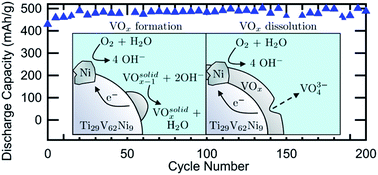
We report the successful development of Ti29V62−xNi9Crx (x = 0, 6, 12) body centered cubic metal hydride (MH) electrodes by addressing vanadium corrosion and dissolution in KOH solutions. By identifying oxygen as the primary source of corrosion and eliminating oxygen with an Ar-purged cell, the Cr-free Ti29V62Ni9 alloy electrode achieved a maximum capacity of 594 mAh g−1, double the capacity of commercial AB5 MH electrodes. With coin cells designed to minimize oxygen evolution, the cycle stability of a Ti29V62Ni9 alloy electrode was greatly improved with either vanadate ion additions to the electrolyte or Cr-substitution in the alloy. Together, both approaches resulted in a reversible capacity of around 500 mAh g−1 for at least 200 cycles. We performed energy density calculations for a 100 W h MH–air cell utilizing the high capacity Ti29V62−xNi9Crx electrodes and found that these cells are comparable in energy density to state-of-the-art Li-ion batteries.


 Please wait while we load your content...
Please wait while we load your content...