A new layered titanate Na2Li2Ti5O12 as a high-performance intercalation anode for sodium-ion batteries†
Abstract
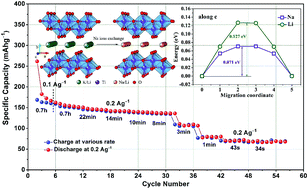
Currently, it is a great challenge to find suitable electrode materials for sodium-ion batteries (SIBs) with large capacity, long cycle life, and high rate capability. Herein, we report a new layered titanate, Na2Li2Ti5O12 (NLT), derived from K2Li2Ti5O12 (KLT) via an ion-exchange method as a SIB anode material. KLT is prepared by a low-temperature solid-state reaction and then transformed into NLT by replacing potassium with sodium in a NaCl solution at room temperature. NLT provides a sodium-ion intercalation voltage at ∼0.5 V versus Na/Na+ and a reversible capacity of 175 mA h g−1 at a current density of 100 mA g−1. It also shows a high sodium-ion diffusion coefficient of 3.0 × 10−10 cm2 s−1, ensuring a high rate capability. For NLT, extremely high discharging rate capability is achieved with a capacity of more than 80 mA h g−1 at a 60 second full discharge and even with 70 mA h g−1 at a 34 second charge. Kinetics analysis based on cyclic voltammogram reveals a typical sodium-ion intercalation behavior in NLT. Furthermore, the first-principle calculation shows a lower migration energy barrier for sodium ions in NLT than that in other layered titanates. These results suggest that NLT is a very promising anode material for high-performance SIBs, especially for fast-charging stable SIBs.


 Please wait while we load your content...
Please wait while we load your content...