Lithium ion conductivity in Li2S–P2S5 glasses – building units and local structure evolution during the crystallization of superionic conductors Li3PS4, Li7P3S11 and Li4P2S7†
Abstract
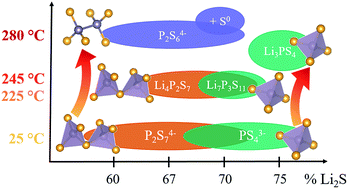
Motivated by the high lithium ion conductivities of lithium thiophosphate glasses, a detailed study is performed on the local chemical nature of the thiophosphate building units within these materials. Using Raman and 31P MAS NMR (Magic Angle Spinning – Nuclear Magnetic Resonance) spectroscopy, the continuous change from dominant P2S74− (di-tetrahedral) anions to PS43− (mono-tetrahedral) anions with increasing Li2S fraction in the (Li2S)x(P2S5)(100−x) glasses is observed. In addition, synchrotron pair distribution function analysis (PDF) of synchrotron X-ray total scattering data is employed to monitor in situ crystallization and phase evolution in this class of materials. Depending on the composition, different crystalline phases evolve, which possess different decomposition temperatures into less conducting phases. The results highlight the critical influence of the local anionic building units on the cation mobility and thermal stability, with PS43− tetrahedra forming the most thermally robust glass ceramics with the highest ionic conductivity.
- This article is part of the themed collection: 2017 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...