Synthesis and oxygen evolution reaction (OER) catalytic performance of Ni2−xRuxP nanocrystals: enhancing activity by dilution of the noble metal†
Abstract
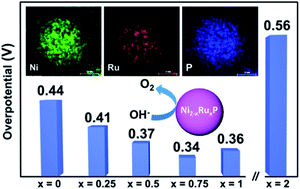
Aiming to create an efficient, less-expensive catalyst for the oxygen evolution reaction (OER), a synthetic protocol is developed to prepare ternary metal phosphide nanoparticles, Ni2−xRuxP, incorporating Ru, a traditional catalyst for OER, and Ni, a highly active but inexpensive metal. Using solution-phase arrested-precipitation reactions, crystalline Ni2−xRuxP particles could be realized for compositions up to x ≤ 1, whereas more Ru-rich compositions, including Ru2P, were amorphous. For x ≤ 1, particles are spherical, of sizes that vary between 5 and 10 nm in diameter (with a clear decreasing trend as the Ru amount is increased), and samples exhibit narrow size distributions (polydispersity < 15%). In contrast, amorphous Ru-rich phases exhibit worm-like morphologies. ICP-MS data indicate the actual metal ratio closely follows the target ratio employed in the synthesis. OER electrocatalytic activity was evaluated for selected compositions over the entire synthesis range (0 ≤ x ≤ 2). Intriguingly, Ru2P proved to be the least active phase (overpotential of 0.56 V at 10 mA cm−2 in 1.0 M KOH) with the best performance observed for the bimetallic Ni1.25Ru0.75P phase (overpotential of 0.34 V). The augmented activity at x = 0.75 is attributed, at least in part, to electronic activation of Ni by Ru, facilitating Ni oxidation and thus decreasing the kinetic barrier for OER.


 Please wait while we load your content...
Please wait while we load your content...