Bromine and nitrogen co-doped tungsten nanoarrays to enable hydrogen evolution at all pH values†
Abstract
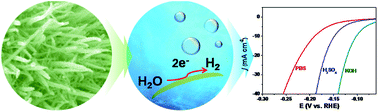
Introducing bromine and nitrogen into metallic tungsten nanoarrays produces a W/BrN electrocatalyst with high performance for the hydrogen evolution reaction (HER) at all pH ranges. Unlike the acid soluble metals (such as Fe, Co, Ni), the resultant W/BrN has successfully inherited the excellent corrosion resistance from pure metallic W, particularly in proton exchange membrane compatible acid electrolytes. However, more importantly, the W/BrN develops some outstanding electrochemical properties, such as enhanced electrical conductivity, optimized electronic properties, and large turnover frequency (TOF), after codoping with Br and N. Hence, the HER performance of W/BrN was higher by several orders of magnitude in current density than that of pure metallic W. To attain current density of 10 mA cm−2, the W/BrN only needs overpotentials of 148, 190 and 94 mV in acidic, neutral and alkaline conditions, respectively. In addition, we investigated the long-term stability of W/BrN in three electrolytes.


 Please wait while we load your content...
Please wait while we load your content...